cryoTEM (or simply cryo-electron microscopy - cryoEM) is a buzzword that encompasses the field of structural biology where the principle experimental technique is transmission electron microscopy (or TEM) followed by extensive image processing. The "cryo" designation in cryoTEM arises from the observation made in the 1980's that the extensive radiation damage to unstained biological materials due to the electron beam can be lowered to manageable levels by holding the specimen near liquid N2 temperatures and using "minimal dose imaging techniques" during the image recording process. These innovations have allowed structural biology using the transmission electron microscope to begin to fulfill the promise of near atomic resolution structures of biological macromolecules, starting with thin protein crystals such as bacterio-rhodopsin (PDB ID 1BRD) and more recently extending to such macro-molecular assemblies as ribosomes, GroEL (PDB ID 3CAU) and some icosahedral viruses that have been imaged under near native conditions using rapid vitrification in biological buffers.
cryo-Transmission Electron Microscopy (cryoTEM)
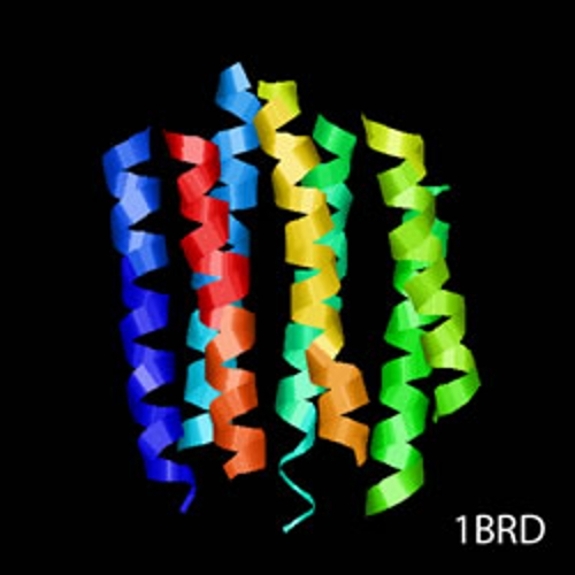
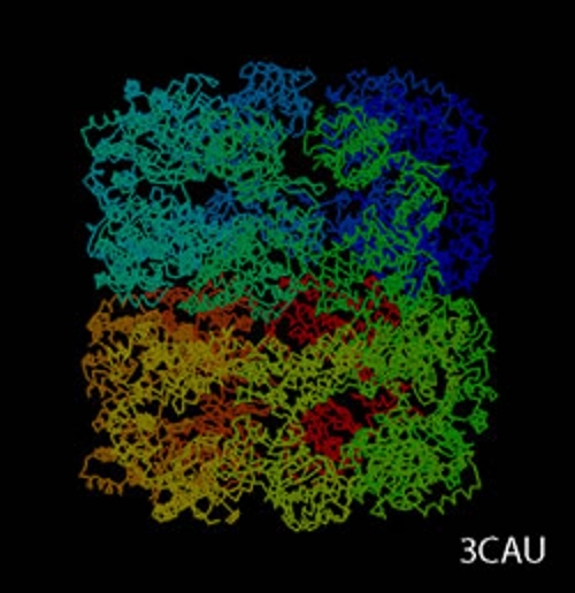
cryoTEM is often a synonym for the electron microscopy of frozen, hydrated specimens and several things have happened in the decades since the initial observations about low temperature effects that have expanded this field from the domain of a select number of specialized labs to a more general and widely practiced area:
- high-quality, commercially-available cryo-holders (e.g., our Gatan 626) and plunge freezers (e.g., our FEI Vitrobot Mk3) have eliminated the need to build such devices in local machine shops and have vastly increased the number of users who can routinely freeze (vitrify) and examine biological specimens at liquid N2 temperatures;
- many modern electron microscopes have standard features such as minimal dose exposure settings and anti-contamination devices that are designed for use with low temperature imaging conditions and commercial cryo-holders and that allow routine observation of specimens at low temperatures;
- commercially produced electron microscopes that operate either at liquid He temperatures or at both liquid N2 and liquid He temperatures have become available that have allowed temperature vs radiation damage studies to extend to much lower temperatures;
- image processing software and expertise in the area of single particle book coveranalysis (see below) has become more accessible and allowed non-experts entry into the somewhat arcane field of cryoTEM.
At the same time that it has become easier and easier to work with vitrified specimens at low temperatures, the term cryoTEM has been expanded to include most structural biology using electron microscopy whether or not vitrified specimens or even low temperatures are involved. A more formal description of this general field of structural biology might be "electron crystallography of biological macromolecules" which is the title of a recent book that gives an excellent overview of the entire field. However, cryoTEM (or cryoEM) seems to be the more commonly used designation and is certainly easier to remember and say.
The experimental details of an investigation using cryoTEM are dictated by the macromolecular order and the symmetry of the system being examined and can be fundamentally divided into the following three sub-areas: two-dimensional crystallography, helical analysis of protein fibers, and the study of isolated macromolecules.
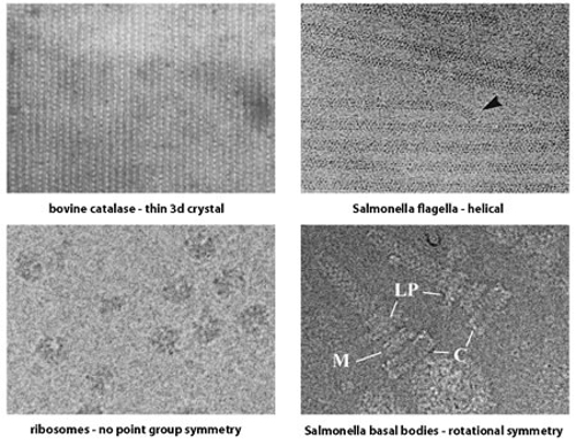
The first two areas involve the application of diffraction theory to TEM images and are more related to each other than to the third area. However, crystallographic diffraction and helical diffraction are rather different and it is useful to divide the diffraction-based approaches into distinct sub-areas.
The third sub-area (also referred to as "single particle analysis") can be further sub-divided based on the three-dimensional point group symmetry present in the macromolecule: specific techniques have been developed for the analysis of icosahedral complexes (532 point group symmetry) such as virus particles and certain enzyme complexes that are rather different from the techniques used for lower or absent point group symmetry. There are also several new developments in the analysis of helical protein fibers that more properly belong to the sub-area of single particle analysis than to the diffraction-based methods mentioned above.
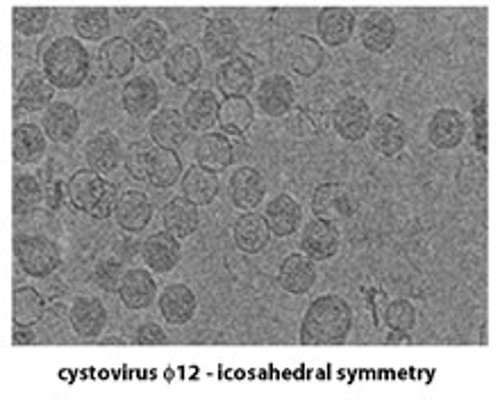
A very recent development in this general area is that the field of biological electron tomography (both with room temperature samples embedded in plastic resins and frozen samples that have been vitrified in different ways) has come to be included under the general umbrella of cryoTEM. This development has been triggered mainly by the wide availability of robust and dependable tomographic data collection software for all recently purchased TEMs, and while not everyone who works in electron tomography also works in the general area of cryoTEM (electron crystallography of biological macromolecules!), it does seem that almost every lab that is devoted to cryoTEM has recently become involved in electron tomography to some extent.
It is also very important to note that while the term "cryoTEM" equates to "structural biology using the electron microscope," the actual work done with the microscope includes conventional transmission electron microscopy (TEM), energy filtered transmission electron microscopy (EFTEM), and electron diffraction (ED) and is likely to expand to include areas such as scanning transmission electron microscopy (STEM), electron energy loss spectroscopy (EELS), and energy-dispersive x-ray spectroscopy (EDS, EDX, or XEDS) that are usually reserved for the materials science community.

